
Draw the Lewis dot structure for SO3 Brainly.in
This chemistry video explains how to draw the Lewis structure of SO3 - Sulfur Trioxide. It discusses the molecular geometry, bond angle, hybridization, and.
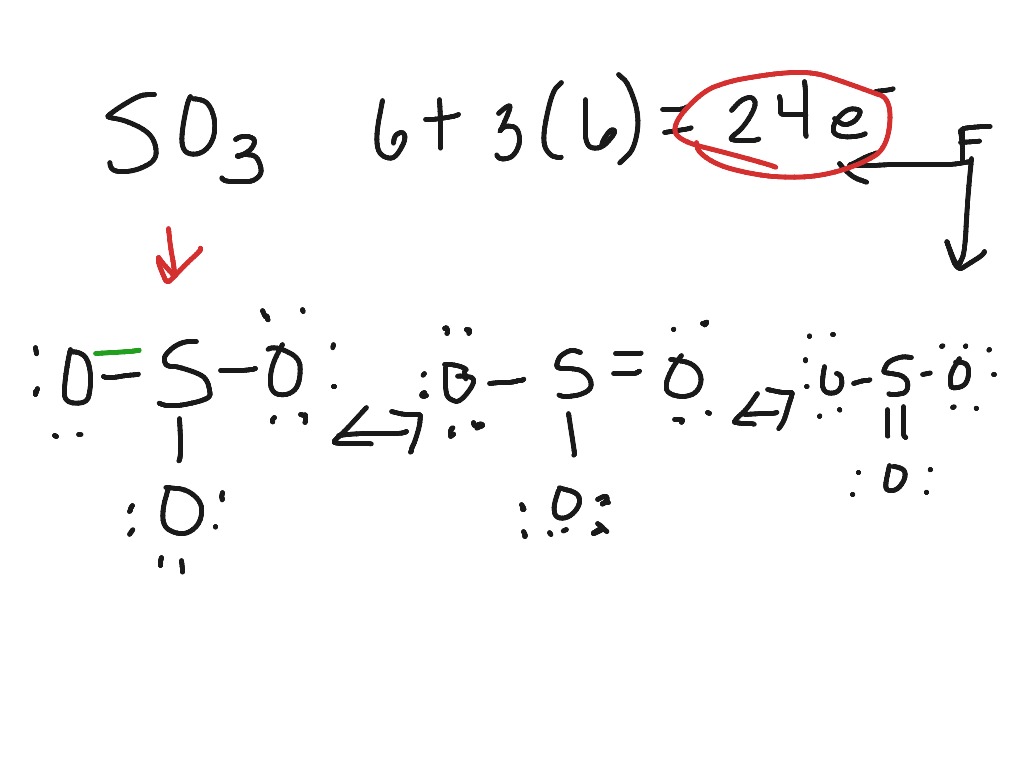
Lewis Dot Diagram For So3 Wiring Diagram
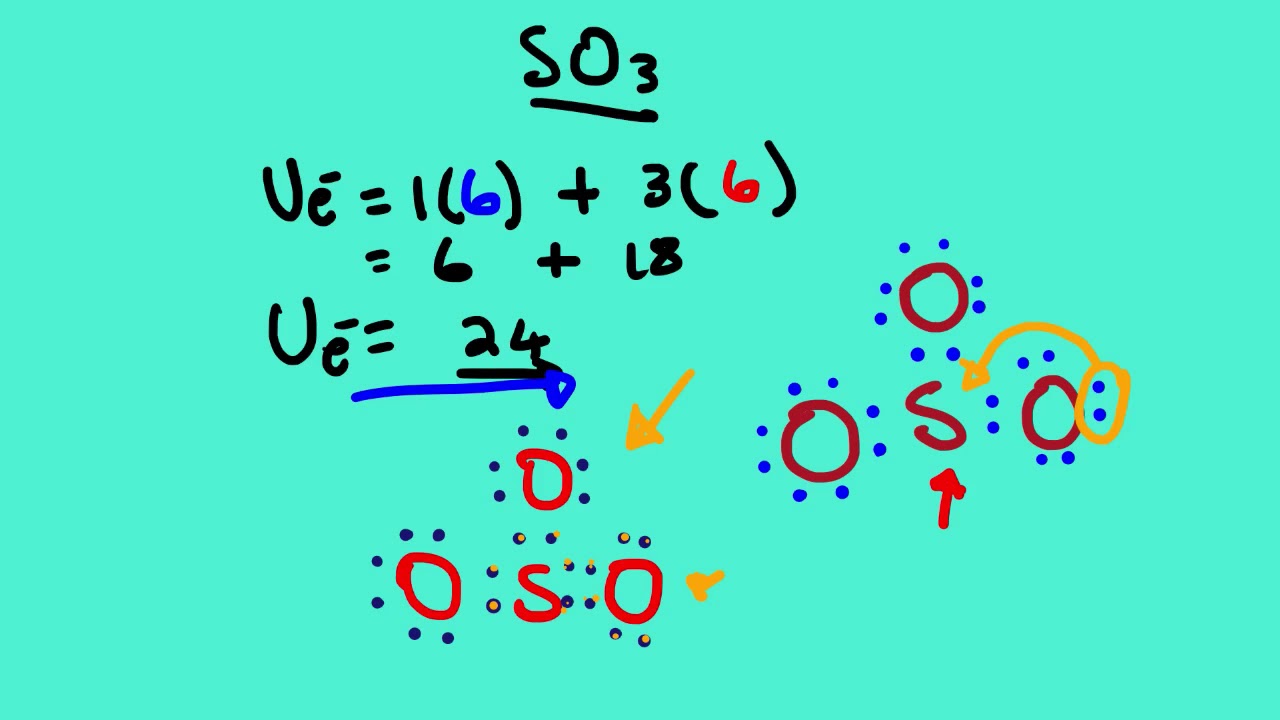
Sulfur brings 6, and oxygen brings 3 each. That means; SO3 has 24 valence electrons. 6 + (3 x 6) = 24. Now have a look of Lewis Structure again; When we draw it, firstly we get the three structures at the top. Sulfur in the center and Oxygen around it is making a connection (each) to the central atom. There should be single bonds initially.
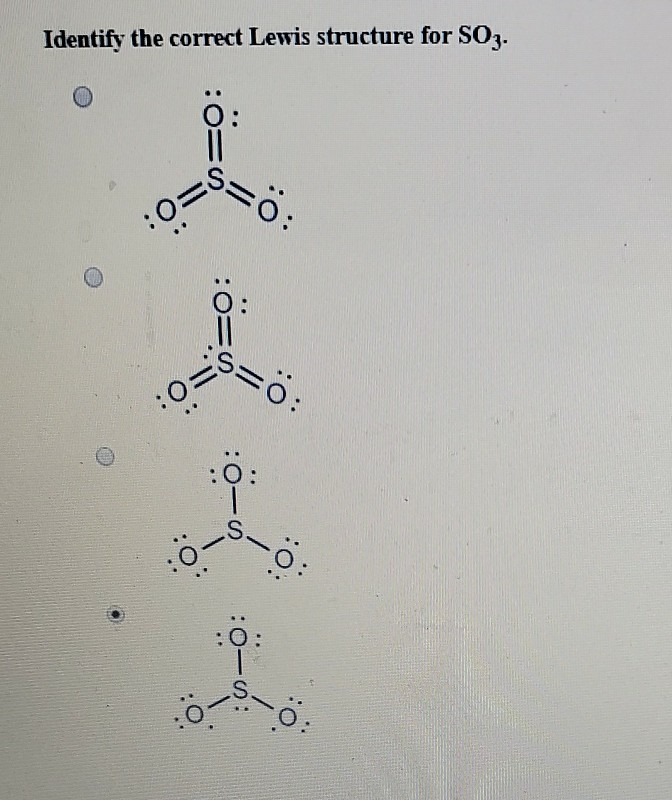
Solved Identify the correct Lewis structure for SO3.
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number.
Simple Procedure for writing Lewis Structures Lewis Structures for
The first step is to sketch the Lewis structure of the SO3 molecule, to add valence electrons around the sulfur atom; the second step is to add valence electrons to the three oxygen atoms, and the final step is to combine the step1 and step2 to get the SO3 Lewis Structure.

Lewis Dot Diagram For So3 Drivenheisenberg
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
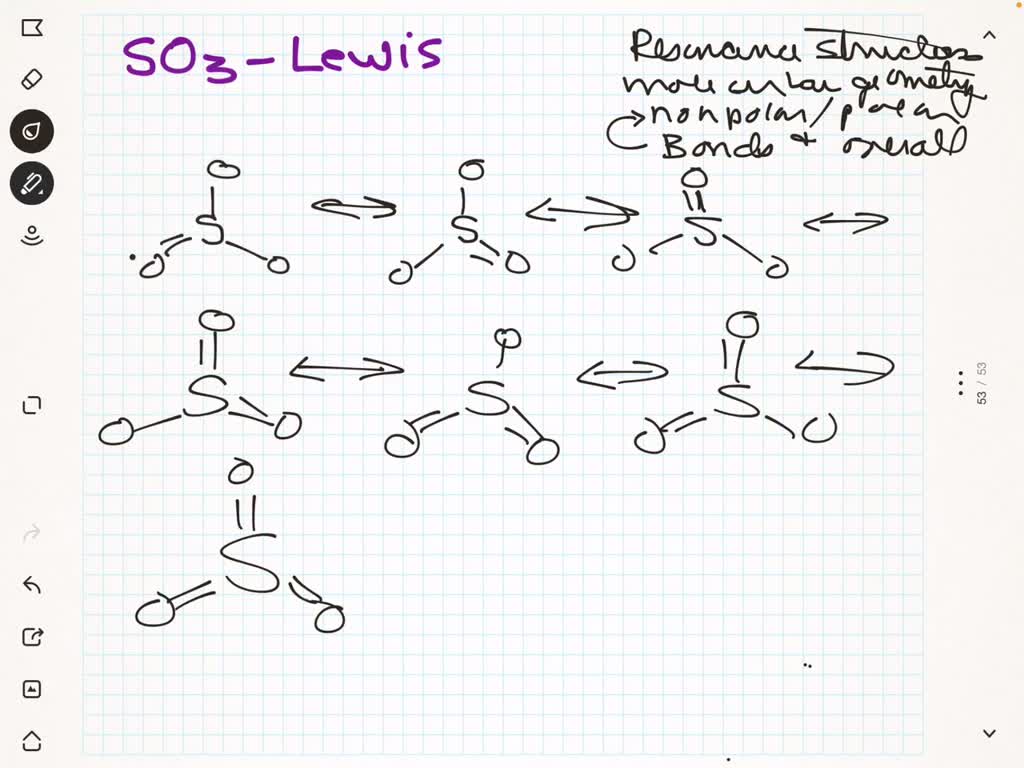
SOLVED a. Give the Lewis electron dot structure for SO3. If there are
The Lewis structure for SO 32- is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 3 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.

michiganswebdesigners Sif6 2 Lewis Structure
How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check.

SO3 Lewis Structure, Molecular Geometry, and Hybridization
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
[Solved] Draw a Lewis Dot structure of SO3. Show ALL resonance
Wayne Breslyn 724K subscribers Join Subscribe Subscribed 1.9K Share 411K views 10 years ago A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3.

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
SO3 lewis structure has a Sulfur atom (S) at the center which is surrounded by three Oxygen atoms (O). There are 3 double bonds between the Sulfur atom (S) and each Oxygen atom (O).. In the above lewis dot structure of SO3, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following.

Draw the Lewis dot structure for SO3 Brainly.in
The lewis structure is also called an electron dot structure which determines the number of valence electrons present in an atom. Moreover, they also describe how these valence electrons are participating in the bond formation to form a molecule.
LEWIS DIAGRAM FOR SO3 YouTube
Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair while all the three Oxygen atoms have 2 lone pairs.
SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur
Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid.

Lewis Dot Structure of SO3 (Sulfur Trioxide) YouTube
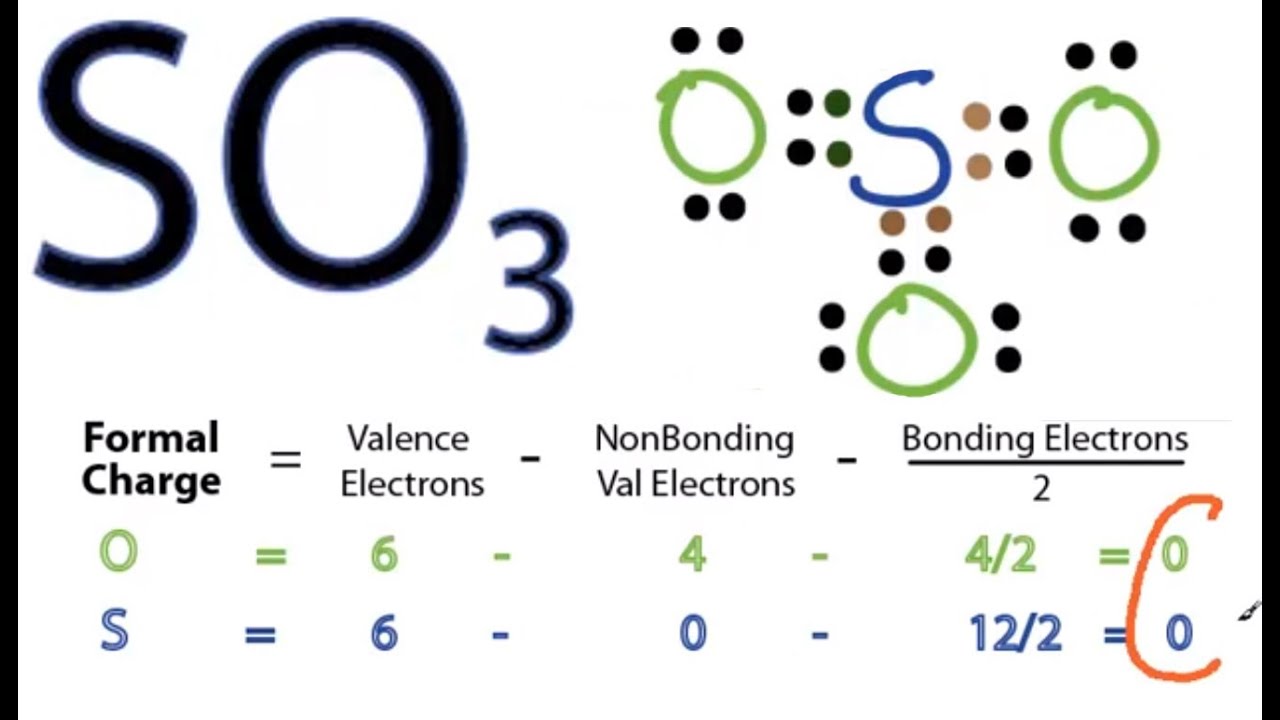
SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 . Video: Drawing the Lewis Structure for SO3 It is helpful if you: Try to draw the SO 3 Lewis structure before watching the video.

Lewis Dot Structure For So3 slidesharedocs
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
Lewis Dot Structure For So3 slidesharedocs
Lewis structure of SO 3 molecule There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Each oxygen atom has two lone pairs in SO 3 lewis structure. But, there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2. Hybridization of SO 3 molecule All atoms have sp 2 hybridization.