
Valence Electrons Definition, Examples, Chart, Periodic Table
The electrons present in the outermost shell of an atom are referred to as valence electrons. The count of these valence electrons determines the valency of an atom. For elements in the s-block and p-block of the periodic table, valency is calculated as the number of valence electrons or eight minus the number of valence electrons.

Periodic trend of valency Chemistry Khan Academy YouTube
Valency is the combining capacity of an element. It is always a whole number. It has no plus or minus sign. The electrons present in the outermost shell of an atom are known as 'Valence Electrons'. We can say valency is the number of electrons an element can lose or gain to attain stability.
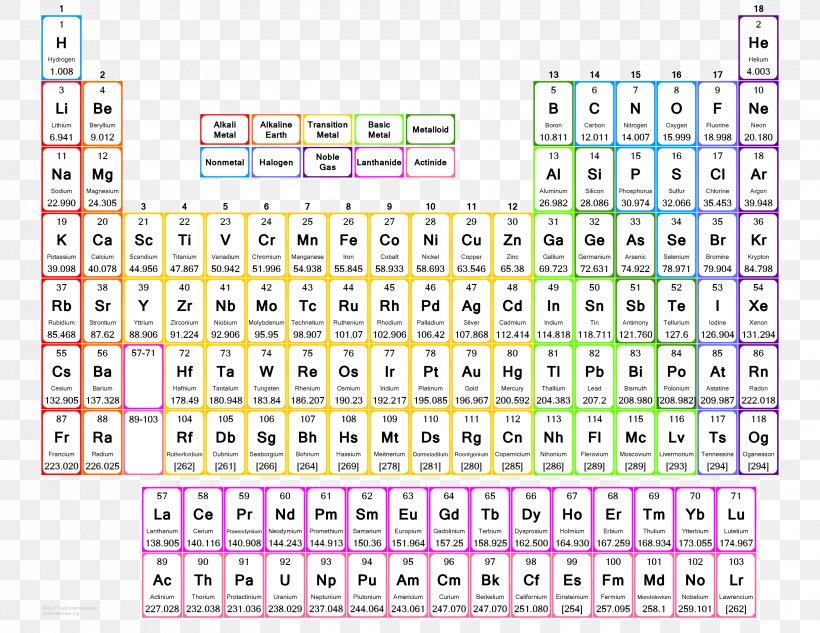
Periodic Table Of Elements With Atomic Mass And Valency Bruin Blog
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given element typically forms.

valency table Scribd india
The valency of an element is a measure of its combining capacity and can be defined as the number of electrons that must be lost or gained by an atom to obtain a stable electron configuration. What does the term 'Oxidation State' mean? The oxidation state of an atom is the number of electrons lost or gained by it.
Find Various Types of Valency of Elements Valencies of 118 Elements
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences.
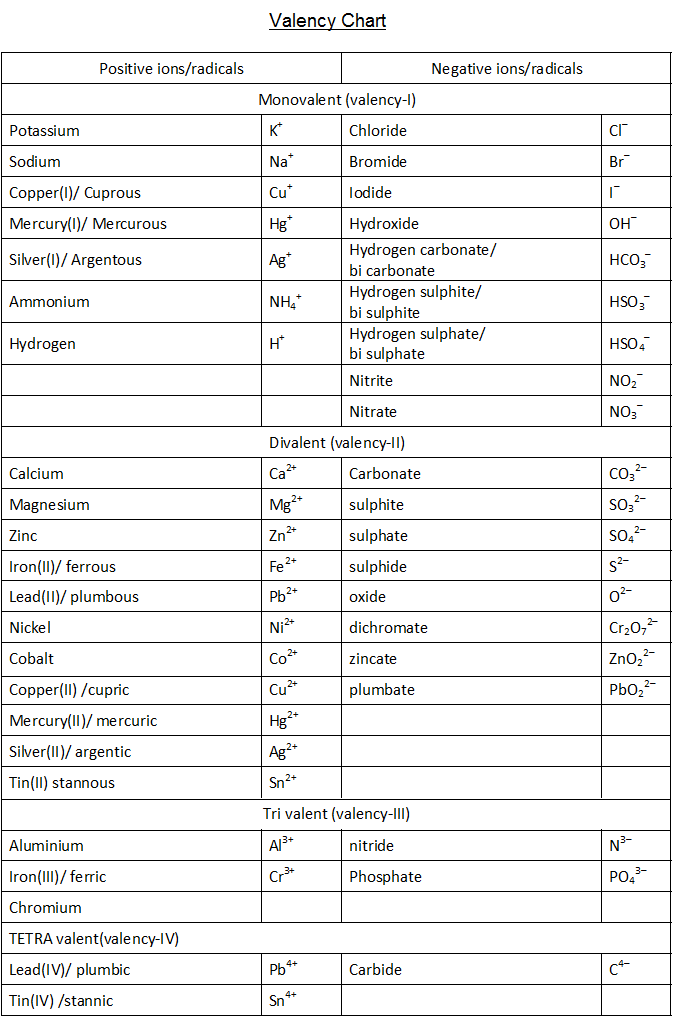
VALENCY CHART
Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Introduced in 1868, the term is used to express both the power of combination of an element in general and the numerical value of the power of combination. A.

Valence Electrons Chart Chemistry Pinterest Chart, Chemistry and Periodic table
Valence is the number of connections an atom tends to form. H is defined to have a valence of 1. For instance: methane, CH 4: carbon atoms have a valence of 4. water, H 2 O: oxygen has a valence of 2. lithium oxide, Li 2 O: lithium has a valence of 1. hydrogen sulfide, H 2 S: sulfur has a valence of 2. aluminum oxide, Al 2 O 3: aluminum has a.
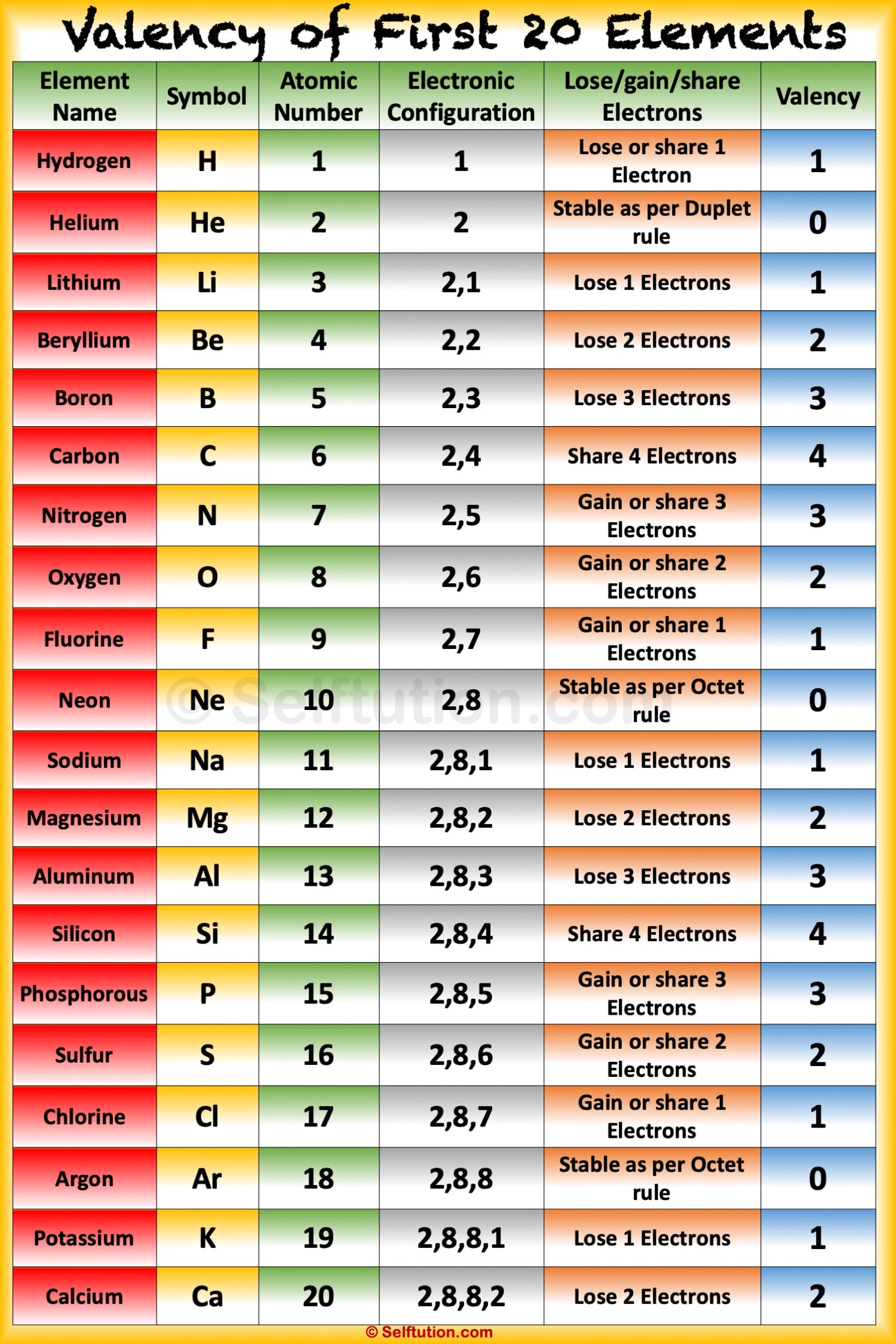
Valency and Variable Valency Valence Shell and Electrons » Selftution
This element Valency PDF is a downloadable version of the Valences of the Elements table. Download PDF As in the table, the most common valences are in BOLD text where values in italics are theoretical values based on periodic table trends. This table requires three sheets of paper to print.

ICSE Class 9 Chemistry Additional Charts Reference Valency Chart PDF
Valence is a measure of how readily an atom or radical forms a bond. Jason Reed / Getty Images By Anne Marie Helmenstine, Ph.D. Updated on September 30, 2018 The words valence and valency have two related meanings in chemistry. Valence describes how easily an atom or radical can combine with other chemical species.

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio How to tell how many
Valency is the combining power of an element. Elements in the same group of the periodic table have the same valency. The valency of an element is related to how many electrons are in the.

Free Printable Periodic Table (With names, charges & Valence Electrons) [PDF] Printables Hub
Hence we assign a valence of 1 to H and to Cl. The valence of O is twice as great, and so we assign a value of 2. Example 4.4.1 4.4. 1: Formula Predictions. Use the data in the first table to predict what formula would be expected for a compound containing (a) sodium and fluorine; (b) calcium and fluorine.

printable periodic table with valence charges printable periodic table with valence charges
Valency is a fundamental concept in chemistry that describes an element's ability to combine with other elements and form compounds. The Valency For All the Elements determines the number of electrons it gains, loses, or shares when forming chemical bonds. This paragraph will provide an overview of the valency for all the elements in the periodic table, highlighting the diversity of chemical.
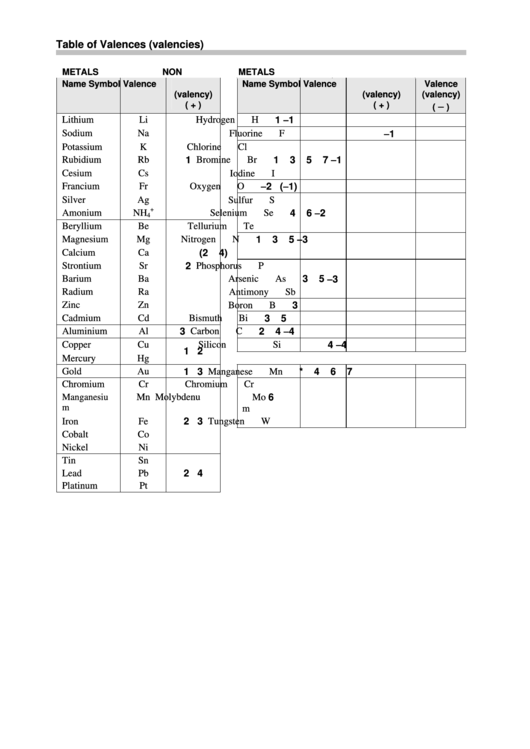
Chemical Table Of Valences (Valencies) printable pdf download
2) Using the Periodic Table. In this method, valency is calculated by referring to the periodic table chart. For example, all the metals, be it hydrogen, lithium, sodium and so on, present in column 1 have valency +1. Similarly, all the elements present in column 17 have valency -1 such as fluorine, chlorine, and so on.

Valency Table Chemical Compounds Periodic Table
The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can use the periodic table to quickly determine the number of valence electrons for main group elements. Valence electrons on the periodic table
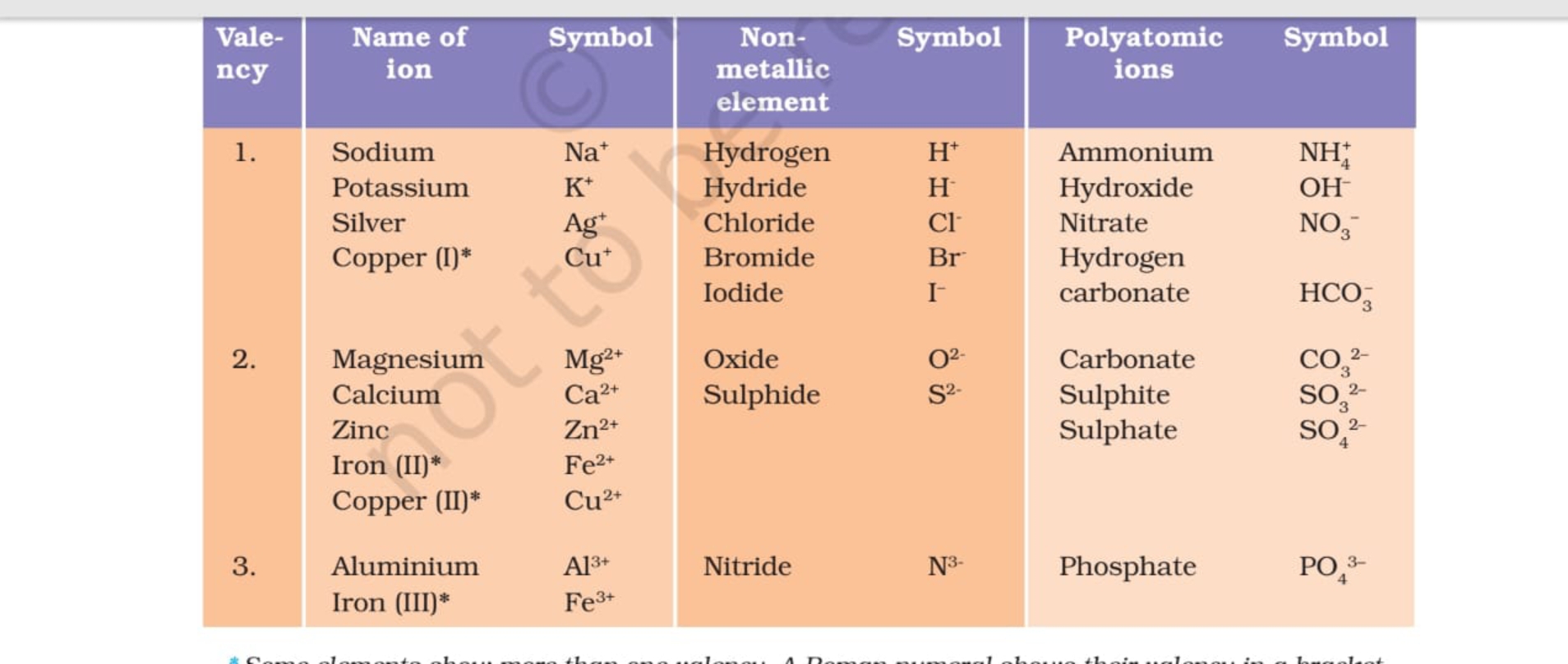
Valency Table Science Notes Teachmint
A valency chart is a diagram that illustrates how many atoms of a particular element are bonded to each atom of another element in a molecule. The valency of an element is the number of bonds it can form. The valency chart determines the molecular formula of a compound. Fill Out the Form for Expert Academic Guidance! Target Exam +91
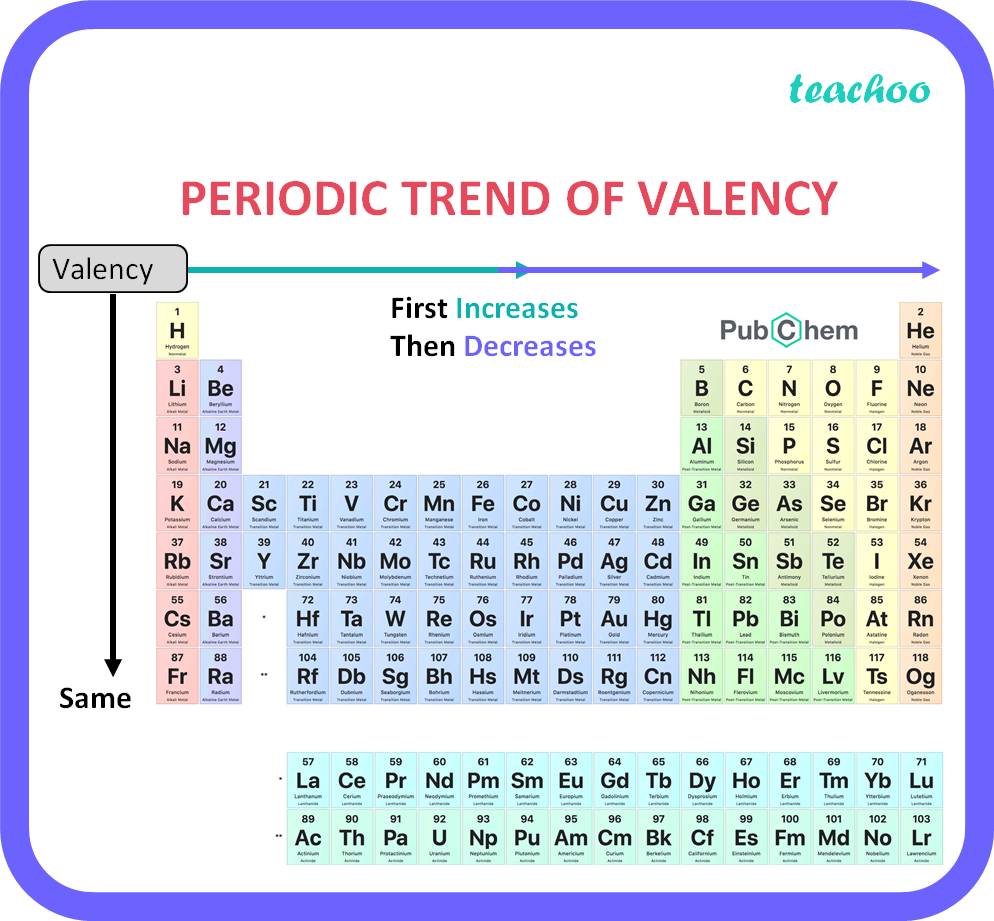
How does valency of an element vary across a period Class 10 Teachoo
The valency chart is a table that shows how many bonds an atom can form with other atoms. The table is arranged so that each row corresponds to an element, and each column corresponds to a valence level. When reading the valency chart, it is important to remember that atoms can only form as many bonds as they have electrons in their outer shell.